How can we help you?
Please get in contact with us to find out more about ECFP and whether we can help you.
Water-in-oil emulsions are found in a range of formulations from agrochemicals to cosmetics, but a key challenge is controlling their stability. When another ingredient is dissolved in the water, disruption of the microstructure can occur due to “compositional ripening”, where water migrates towards and swells solute rich droplets, leaving other droplets to shrink. This is a key challenge in creating reduced calorie foods by replacing oil with water droplets. A new study recently published in Langmuir and led by Dr Raj Tadi with Prof. Paul Clegg shows how modifying the interactions between solid particles used to stabilise the emulsions can influence the fate of the shrinking droplets. This points towards mechanisms to control and manipulate compositional ripening.
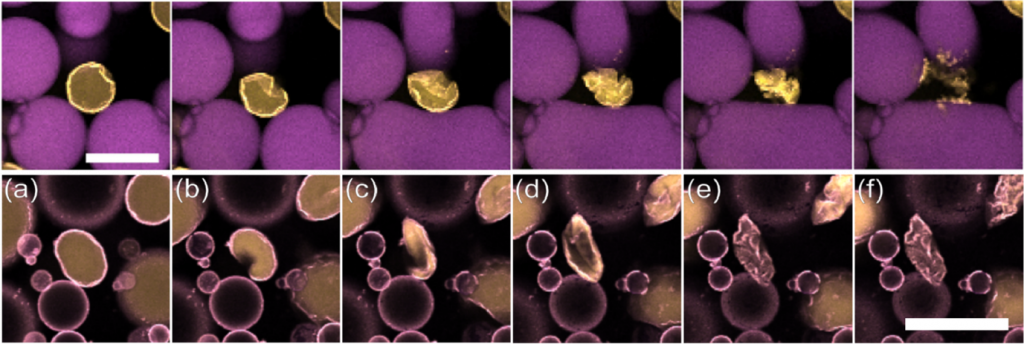
Conventional oil-in-water emulsions can be stabilised by the addition of small, hard colloidal particles that adsorb to the interface and prevent coalescence in a “Pickering emulsion”. However, the strong osmotic pressure created by dissolved ingredients, such as sugar, can cause solute-poor drops to shrink and ultimately the particle coating to explode (top row of images above, scale bar 50 µm, from Figure 5 in Tadi et al. 2023 republished here under a CC-BY 3.0 licence). Adding a solvent to the oil phase that can partially swell – and potentially soften – the particles instead means droplets just slowly crumple (bottom row of images above, scale bar 100 m, from Figure 2 in Tadi et al. 2025 republished here under a CC-BY 4.0 licence). This demonstrates how particle layers can be made tougher and more resilient.
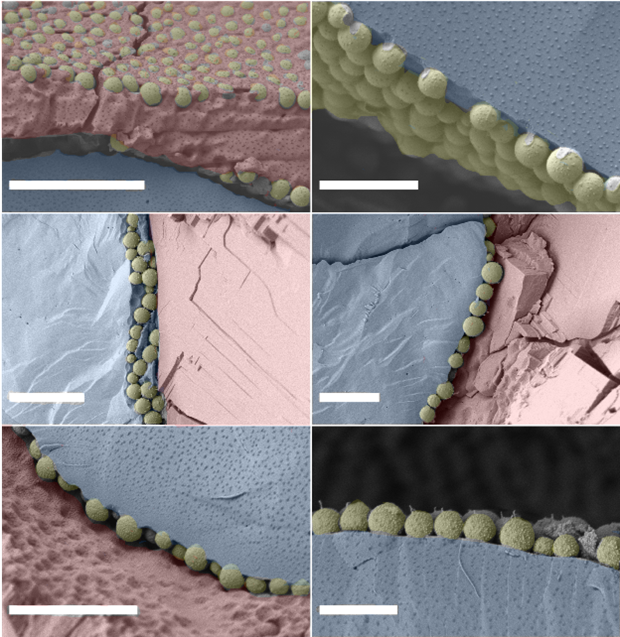
To understand the mechanism, high-resolution cryogenic scanning electron microscopy (cryo-SEM, Dr Fraser Laidlaw) images (above) were taken to show that the initial particles (top) remain solid and distinct after the addition of toluene (middle) and when the oil mixture is renewed with pure dodecane (bottom). Instead, the change in response comes from alteration of the short-range interactions, which were probed using a novel contactless interfacial rheology method developed by Dr James Richards and Dr Job Thijssen that showed a stretchier particle network form.
This work on well-controlled model systems demonstrates how emulsion stability can be understood and controlled in complex, multi-component formulations and was supported by a BBSRC Mondelēz International CTP Studentship co-supervised by Dr Tom Curwen and Dr Beth Green.
Please get in contact with us to find out more about ECFP and whether we can help you.