How can we help you?
Please get in contact with us to find out more about ECFP and whether we can help you.
Emulsions are dispersions of micron-sized liquid droplets within another liquid. They are found across a wide range of industries, including food and drink (milk, salad dressings, mayonnaise), paints and coatings (emulsion paints, many glues), and pharmaceuticals (topical creams, emulsion adjuvants in vaccines). The liquids used to make emulsions are normally immiscible and energy is needed to create interfaces between the droplets and the bulk liquid phase. Think of a salad dressing that you must shake before serving. Without added stabilisers, it will quickly separate into two distinct layers. However, some systems exhibit spontaneous emulsification where no mixing is needed and the emulsion remains stable for long periods of time. Understanding this phenomenon could help industries develop novel low-energy, surfactant-free emulsification methods.

Ouzo is a clear, aniseed-flavoured spirit, consisting of roughly 60% water, 40% ethanol, and a very small amount of anise oil. Both oil and water are soluble in ethanol, provided it is present in sufficient quantities. When the neat spirit is diluted with water, the drink will turn cloudy due to oil-rich microdroplets precipitating out of the solution. This is commonly known as the ouzo effect and is an example of spontaneous emulsification. Interestingly (and perplexingly), the emulsion exhibits long term stability and stays cloudy for weeks, an important feature for many industrial emulsions. To fully understand and predict the behaviour of such a system, it is crucial to determine its complete equilibrium phase diagram. To date, this has not been done for ouzo.
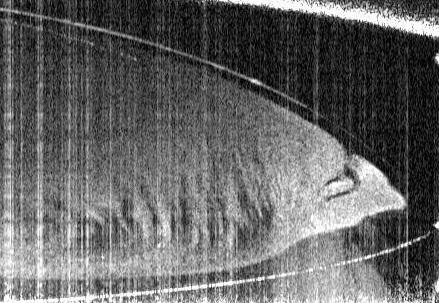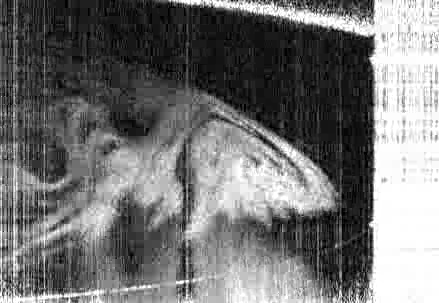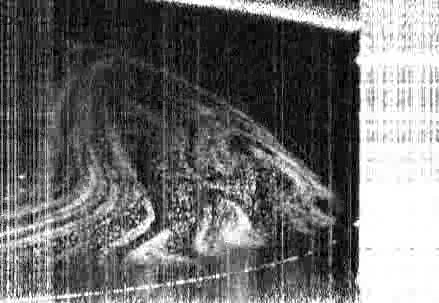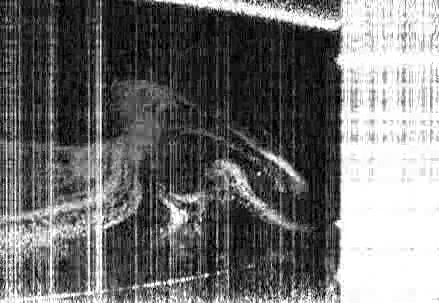
The team took an experimental approach to building a phase diagram for a model system very similar to the commercial ouzo spirit, measuring the phase boundary along with the compositions and physical properties of coexisting phases, including the interfacial tension and density. This experimental approach was verified via the development of a theoretical model using lattice density functional theory, the predictions of which closely matched the experimental findings.
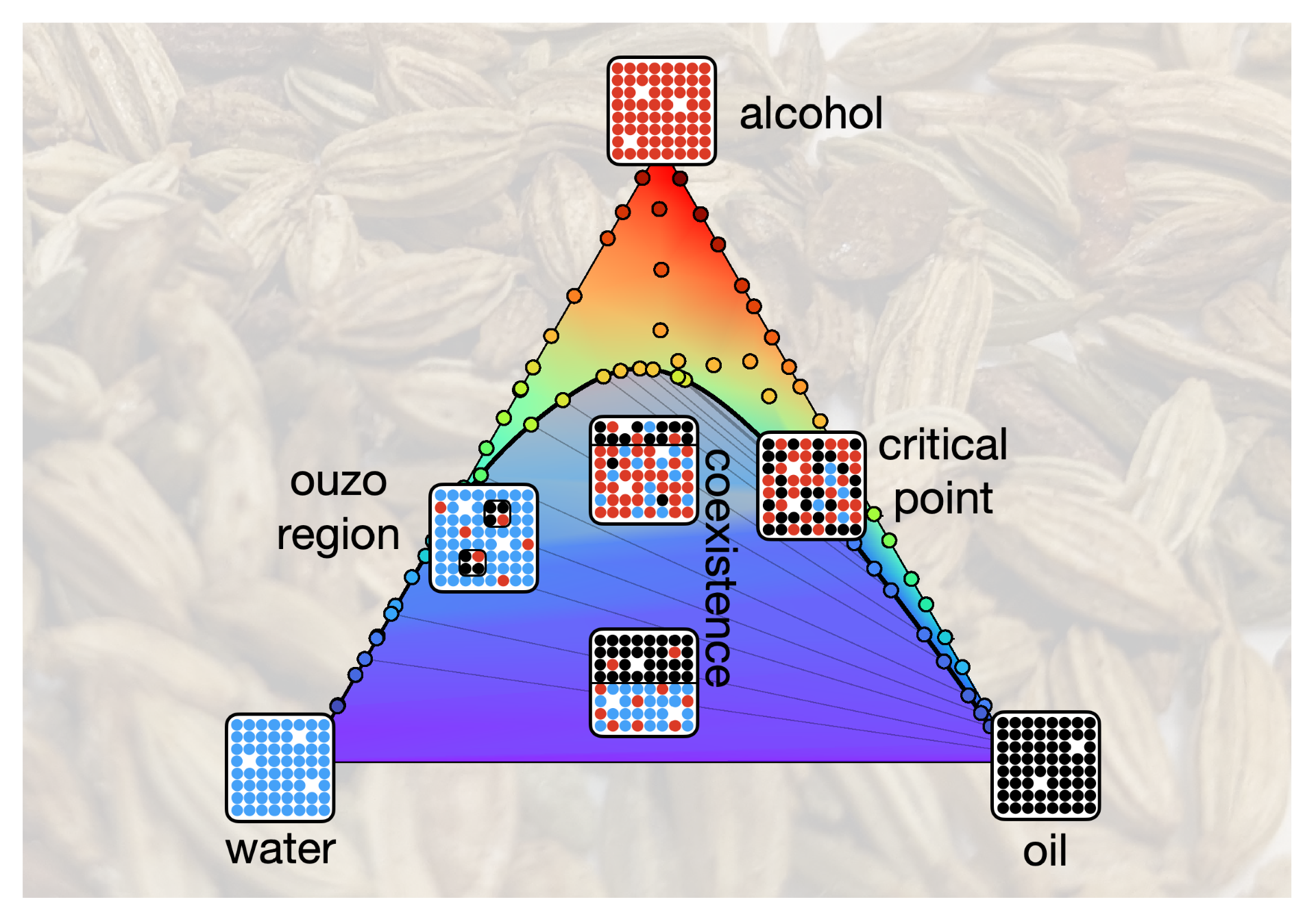
The experimental phase diagram and predictive model developed by the researchers can be taken forward in the future to study the non-equilibrium behaviour of the system, for example its stability. Understanding the mechanism by which this surfactant-free system remains stable could help develop other surfactant-free emulsions. Recently this has been an area of increasing focus for formulation scientists, as industries look to move towards more sustainable formulations and away from the use of petroleum-derived surfactants.
Please get in contact with us to find out more about ECFP and whether we can help you.
ECFP delivers fundamental product insight enabling improved formulation and processing for a more sustainable future.