Drinks, pesticides and inks all have one thing in common – without careful formulation they can all be sensitive to separation and sedimentation, which draws large, dense particles and aggregates to the bottom of the container (or cause low density dispersions to “cream”. This then leaves a visible layer of liquid above the bulk of the product. The resulting appearance often deters customers and can cause havoc on the coatings industry, where a key requirement is to keep a constant concentration of the product during application.
Fujifilm are a world-leading photography and printing company who manufacture high-quality pigment dispersions for use in inks and paints. In order to do so, their chemists develop polymers in-house to ensure that pigment particles remain dispersed for the shelf-life of the product and to enhance in-house printing performance. Through a fundamental understanding of the physical mechanisms underpinning the interactions between ingredients, the company is able to optimise a formulation.

Fujifilm was keen to improve their understanding of the interactions between a new polymer and a pigment particle. They had noticed that a sediment formed unexpectedly at high concentrations and they wanted to understand why.
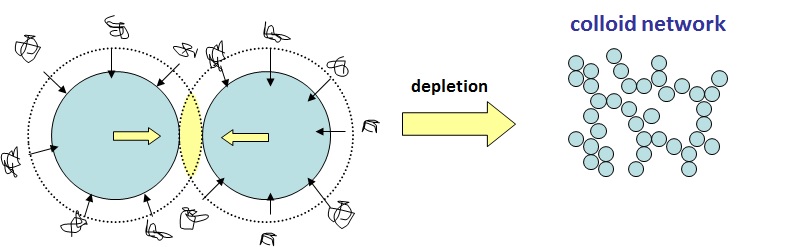
From their knowledge of Soft Matter Physics, ECFP knew that a colloid-polymer system can behave in two different ways: 1) “the bridging mechanism” where sticky polymers can bridge particles, causing aggregation and sedimentation or 2) “the depletion mechanism” where non-absorbing polymers cause larges particles to aggregate through applying an osmotic pressure. To deduce the mechanism that dominated, Dr Matthew Reeves explored concentration space to find the signature behaviour indicating the dominant mechanism. He was guided by Professor Wilson Poon, who spent >20 years studying the depletion mechanism – where “big bits” aggregate in the presence of “small bits” such as non-absorbing polymers, micelles or nanoparticles. With the knowledge gained the company is able to produce stable dispersions with confidence.
Please get in contact with us to find out more about ECFP and whether we can help you.

Case study – Quorn Foods
ECFP delivers fundamental product insight enabling improved formulation and processing for a more sustainable future.